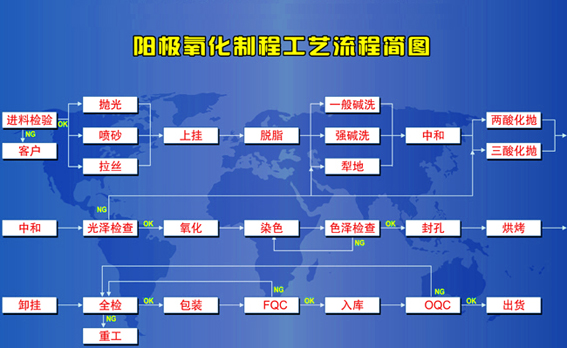
1. Surface pretreatment No matter what method is used to process aluminum and products, there will be varying degrees of dirt and defects on the surface, such as dust, metal oxide (alumina film formed at natural or high temperature), and residual oil , Asphalt signs, manual handling fingerprints (the main components are fatty acids and nitrogen-containing compounds), welding flux and corrosion salts, metal burrs, minor scratches, etc. Therefore, before the oxidation treatment, the surface of the product must be cleaned by chemical and physical methods to expose the pure metal matrix to facilitate the smooth progress of the oxidation and coloring, so as to obtain a firm bond with the matrix, color and thickness to meet the requirements and have The best artificial membrane with good corrosion resistance, wear resistance and weather resistance. (1) Degreasing of degreasing aluminum and aluminum alloy surfaces includes organic solvent degreasing, surfactant degreasing, alkaline solution degreasing, acid solution degreasing, electrolytic degreasing, and emulsifying degreasing. Emulsified solution paraffin triethanolamine oleic acid pine oil water 8.0% 0.25% 0.5% 2.25% 89% room temperature appropriate water cleaning solution composition by volume The organic solvent is degreasing using the characteristics of oils that are easily soluble in organic solvents. Commonly used solvents are gasoline and kerosene , Ethanol, isoamyl acetate, acetone, carbon tetrachloride, trichloroethylene, etc. Organic solvents are only used for the degreasing of small batches or extremely dirty products. Surfactants are substances that can significantly reduce the surface tension of liquids at very low concentrations. Surfactants commonly used for degreasing include soap, synthetic detergent, sodium lauryl sulfate, sodium dodecylbenzene sulfonate and so on. There are many formulations of alkaline degreasing solutions. The traditional process uses sodium phosphate, sodium hydroxide and sodium silicate. Among them, sodium phosphate and sodium silicate have corrosion inhibition, wetting and stabilizing effects. Heating and stirring of the solution helps to obtain the best The degreasing effect. Grease can also undergo hydrolysis in the presence of acid to produce glycerol and corresponding higher fatty acids. Electrolytic degreasing can use anode current, cathode current or alternating current. Cathodic current degreasing in alkaline solution, the anode is preferably nickel-plated steel sheet. It is not commonly used in surface treatment of aluminum and aluminum alloys. The solution used for emulsification degreasing is a two-phase or multi-phase solution composed of mutually insoluble water and organic solvent, and is added with a detergent that reduces surface tension and has affinity for each phase. (2) Alkali Etching Agent Alkali Etching Agent is the process of surface cleaning of aluminum products in sodium hydroxide solution with or without other substances. It is usually called alkali corrosion or alkaline washing. Its function is to be used as a supplementary treatment after the product has been degreasing by some degreasing methods to further clean up the oily dirt attached to the surface; to remove the natural oxide film and slight scratches on the surface of the product. Therefore, the product exposes the pure metal matrix, which is beneficial to the formation of the anode film and obtains a higher quality film layer. In addition, by changing the composition, temperature, processing time and other operating conditions of the solution, the etched surface in different states such as smooth or satin matt or gloss can be obtained. The basic composition of the etching solution is sodium hydroxide. In addition, regulators (NaF, sodium nitrate), scale inhibitors, (gluconate, enanthate, tartrate, acacia, dextrin, etc.) are added, and multivalent Chelating agent (polyphosphate), detergent aluminum surface anodic oxidation treatment method (2) aluminum surface anodic oxidation treatment method (2) (3) neutralization and water cleaning the gray or black ash attached to the surface of aluminum products after etching It does not dissolve in cold or hot water, but it can be dissolved in acidic solution. Therefore, the products that have been etched by hot alkaline solution must be processed to remove ash and residual lye to expose the bright basic metal surface Acid leaching cleaning, this process is called neutralization, gloss or light treatment. The process is that the product is immersed in a solution of 300-400g/L nitric acid (1420kg/m3) at room temperature. The immersion time varies with the metal composition. The general immersion time is 3-5 minutes. The ash on aluminum alloy products containing silicon or manganese can be treated with a mixture of nitric acid and hydrofluoric acid in a volume ratio of 3:1 at room temperature for 5-15 seconds. The neutralization treatment can also be carried out at room temperature in a solution containing 300-400g/L nitric acid and 5-15g/L chromium oxide or 100g/L chromium oxide plus sulfuric acid (1840kg/m3) 10ml/L solution. The purpose of water cleaning between each process is to completely remove the residual liquid and water-soluble reaction products on the surface of the product, so as to prevent the bath liquid in the next process from being polluted, and to ensure the treatment efficiency and quality. Most of the cleaning uses one cold water cleaning. However, the products after alkali etching generally adopt double cleaning of hot water followed by cold water. The temperature of the hot water is 40-60 degrees. The neutralized products can be oxidized after cleaning with water, so this cleaning should be done carefully to prevent contamination of the cleaned surface. Otherwise, the effective treatment of the first few procedures may be lost due to improper final cleaning. Products after neutralization and water cleaning should be oxidized with the above. The staying time in the air should not be too long, such as staying for 30-40 minutes, the product needs to be etched and neutralized again. 2. The natural alumina on the surface of anodized aluminum products is soft and thin, and has poor corrosion resistance. It cannot be an effective protective layer and is not suitable for coloring. Artificial oxide film mainly uses chemical oxidation and anodic oxidation. Chemical oxidation is a process in which part of the base metal reacts on aluminum products in a weakly alkaline or weakly acidic solution to thicken the natural oxide film on the surface or produce some other passive film. The commonly used chemical oxide film is chromic acid film And phosphoric acid film, they are thin and have good adsorption properties, and can be colored and sealed. Table-3 describes the chemical oxidation process of aluminum products. Compared with the anodic oxide film, the chemical oxide film is much thinner, has lower corrosion resistance and hardness, and is not easy to color, and the light resistance after coloring is poor. Therefore, the coloring and color matching of metal aluminum only introduces anodizing treatment. Aluminum surface anodizing treatment method (3) (1) General concept of anodizing treatment
1. The general principle of anodic oxide film formation
The process of using aluminum or aluminum alloy products as anodes in an electrolyte solution and using electrolysis to form an aluminum oxide film on the surface is called anodizing of aluminum and aluminum alloys. The cathode in the device is a material with high chemical stability in the electrolytic solution, such as lead, stainless steel, aluminum, etc. The principle of aluminum anodization is essentially the principle of water electrolysis. When an electric current passes, hydrogen gas is released on the cathode; on the anode, the precipitated oxygen is not only molecular oxygen, but also atomic oxygen (O) and ionic oxygen, which are usually represented by molecular oxygen in the reaction. The aluminum as the anode is oxidized by the oxygen precipitated thereon to form an anhydrous aluminum oxide film. Not all of the oxygen generated interacts with the aluminum, and part of it is precipitated in a gaseous form.
2. Selection of anodizing electrolytic solution
A prerequisite for the growth of the anodic oxide film is that the electrolyte should have a dissolving effect on the oxide film. But this is not to say that anodic oxidation in all electrolytes with dissolving effect can produce oxide film or the properties of the oxide film produced are the same. 3. Types of anodizing
According to the current form, anodizing is divided into direct current anodizing, alternating current anodizing, and pulse current anodizing. According to electrolyte, it is divided into sulfuric acid, oxalic acid, chromic acid, mixed acid and natural color anodizing with sulfo-organic acid as the main solution. According to the characteristics of the film layer, it is divided into: ordinary film, hard film (thick film), porcelain film, bright modified layer, semiconductor barrier layer and other anodic oxidation. Among them, the application of direct current sulfuric acid anodization is the most common. 4. Structure and properties of anodic oxide film
The anodic oxide film is composed of two layers. The thick, porous outer layer is grown on the dense inner layer with dielectric properties. The latter is called the barrier layer (also called the active layer). Observed and studied with an electron microscope, almost all the longitudinal and longitudinal surfaces of the film showed tubular holes perpendicular to the metal surface, which penetrated the outer layer of the film to the barrier layer at the interface between the oxide film and the metal. With each pore as the main axis, dense aluminum oxide forms a honeycomb hexagonal prism called a unit cell. The entire film layer is composed of countless such unit cells. The barrier layer is composed of anhydrous alumina, which is thin and dense, has high hardness and prevents the passage of current. The thickness of the barrier layer is about 0.03-0.05μm, which is 0.5%-2.0% of the total film thickness. The porous outer layer of the oxide film is mainly composed of amorphous alumina and a small amount of hydrated alumina, and also contains cations of the electrolyte. When the electrolyte is sulfuric acid, the sulfate content in the membrane is 13%-17% under normal conditions. Most of the excellent properties of the oxide film are determined by the thickness and porosity of the porous outer layer, which are closely related to the anodizing conditions.
(2) Direct current sulfuric acid anodizing
1. Growth mechanism of oxide film
Anodized in sulfuric acid electrolyte, the surface of aluminum products as anodes is uniformly oxidized during the initial short time of anodization, forming a very thin and very dense film. Due to the effect of sulfuric acid solution, the weakest point of the film ( Such as grain boundaries, impurity dense points, lattice defects or structural deformations) locally dissolve, and a large number of pores, namely primary oxidation centers, appear, so that the base metal can contact the electrolyte entering the pores, and the current can continue to conduct. The generated oxygen ions are used to oxidize the new metal and expand around the bottom of the hole, and finally merge to form a new film between the old film and the metal, making the partially dissolved old film seem to be "repaired" . With the extension of the oxidation time, the continuous dissolution or repair of the film, the oxidation reaction can develop in depth, so that the surface of the product generates an oxide film composed of a thin and dense inner layer and a thick and porous outer layer. The thickness of the inner layer (barrier layer, dielectric layer, active layer) remains basically unchanged until the end of oxidation, but the position continues to move to the depth; the outer layer thickens with time during a certain oxidation time. Aluminum surface anodizing treatment method (four) (three) other anodizing
1. Oxalic acid anodizing
Most of the factors that affect sulfuric acid anodization are also applicable to oxalic acid anodization. Oxalic acid anodization can use direct current, alternating current, or superposition of alternating and direct current. Alternating current oxidation is softer and less elastic than direct current under the same conditions; pitting corrosion is likely to occur when direct current oxidation is used, while alternating current oxidation can prevent it. As the AC component increases, the film’s corrosion resistance increases, but the color darkens , The coloring is worse than sulfuric acid film. The concentration of free oxalic acid in the electrolyte is 3%-10%, generally 3%-5%. During the oxidation process, it consumes about 0.13-0.14g per A?h, and at the same time, 0.08-0.09g aluminum is dissolved in every A?h The electrolyte generates aluminum oxalate, which needs to consume 5 times the amount of oxalic acid. The aluminum ion concentration in the solution is controlled below 20g/L. When it contains 30g/L aluminum, the solution becomes invalid. Oxalic acid electrolyte is very sensitive to chloride. When anodizing pure aluminum or aluminum alloy, the chloride content should not exceed 0.04-0.02g/L. The solution is best prepared with pure water. As the temperature of the electrolyte increases, the film becomes thinner. In order to obtain a thick film, the pH of the solution should be increased. Direct current anodizing uses lead, graphite or stainless steel as the cathode, and its area ratio to the anode is between (1:2) and (1:1). Oxalic acid is a weak acid with low solubility. When aluminum is oxidized, the product and electrolyte must be cooled. The thickness and color of the oxalic acid film vary depending on the alloy composition. The film thickness of pure aluminum is light yellow or silver white, while the film thickness of the alloy is thin and dark, such as yellow and brass. After oxidation, the film is cleaned. If it is not dyed, it can be sealed with steam at a pressure of 3.43×10 4 Pa for 30-60 minutes.
2. Chromic acid anodizing
The chromic acid anodizing process is shown in Table-4. Concentration analysis should be carried out frequently during the oxidation process, and chromic anhydride should be added in due course. The cathode materials for electrolysis can be lead, iron, stainless steel, and the best ratio of anode to cathode area is (5:1)-(10:1). When there are many trivalent chromium ions in the solution, it can be oxidized to hexavalent chromium ions by electrolysis. The sulfate content in the solution exceeds 0.5%, and the anodizing effect is not good. When there are too many sulfate ions, barium hydroxide or barium carbonate can be added to make barium sulfate precipitate. The chloride content in the solution should not exceed 0.2g/L. When the chromium content in the solution exceeds 70g/L, the solution should be diluted or replaced. There are two types of chromic acid anodic oxidation: the anodic oxidation method with periodic voltage changes or the constant voltage anodic oxidation method (rapid chromic acid method).
3. Hard (thick film) anodizing
Hard anodizing is a process for forming thick and hard oxide film on the surface of aluminum and aluminum alloys. The maximum thickness of the hard film can reach 250μm, the microhardness of the film formed on pure aluminum is 12000-15000MPa, and the alloy is generally 4000-6000MPa, which is almost the same as the hard chromium coating. They have excellent wear resistance at low conformance. The porosity of hard film is about 20%, which is lower than that of conventional sulfuric acid film. 4. Porcelain anodizing
Porcelain anodized aluminum and aluminum alloys are anodized in oxalic acid, citric acid and boric acid titanium salt, zirconium salt or thorium salt solution. The salt metal hydroxide in the solution enters the pores of the oxide film, so that the surface of the product shows It is a treatment process with opaque and dense enamel or similar plastic appearance with special luster. Porcelain anodizing treatment process is basically the same as conventional sulfuric acid anodizing. The difference is that porcelain anodizing is performed at high DC voltage (115-125V) and higher solution temperature (50-60 degrees), and the electrolyte is often stirred 、Adjust the pH value frequently to make it in the range of 1.6-2.
Dongguan Zhigao Industrial Co., Ltd. specializes in processing automotive power amplifier aluminum radiators, home power amplifier radiators and LED lamp heat dissipation bodies, and concurrently processes various aluminum products, hardware and electronic accessories, deep drawing products, and heat sinks ( Aluminum, copper), special terminals, etc.